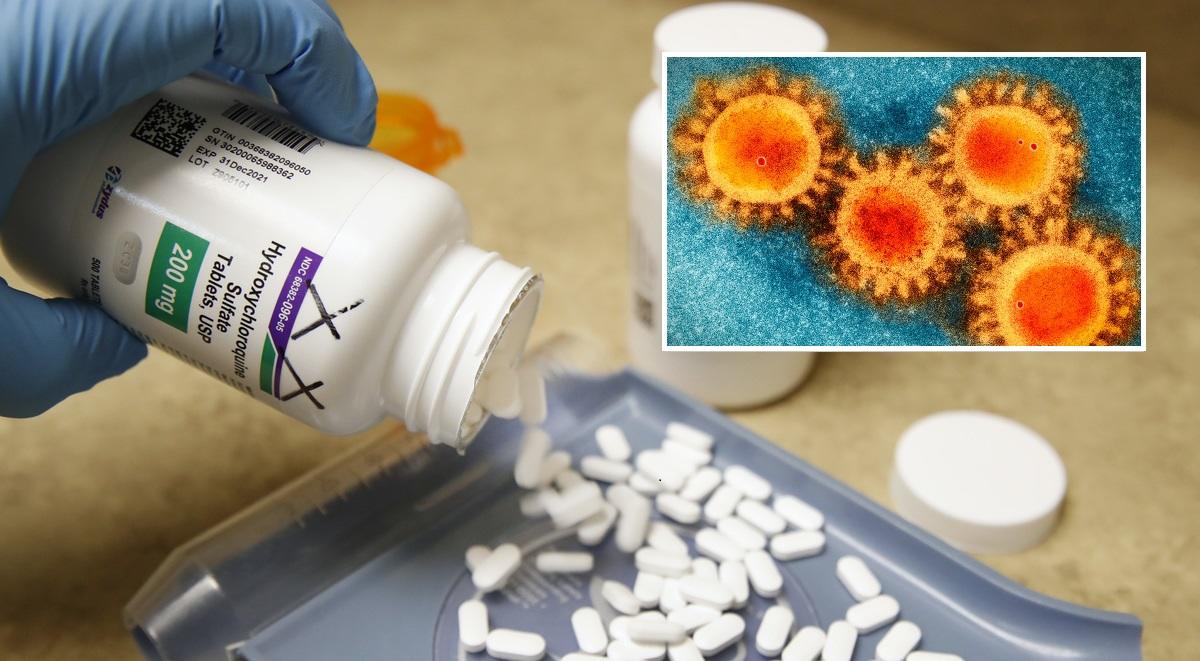
Global Hydroxychloroquine Study To Resume After Positive Trial Results
UK regulators have approved the resumption of a global trial aimed at determining whether hydroxychloroquine and chloroquine are effective in preventing COVID-19 infections in healthcare settings, according to Reuters.
The trial, known as COPCOV, was paused after another British study found the drugs to be ineffective in treating the virus, however the Medicines and Healthcare Products Regulatory Agency (MHRA) has now allowed the research to resume following positive COPCOV trial results.
"Participants will take the study drugs each day for a period of three months, and will be followed closely to see how well the drug is tolerated, whether they contract the virus, and if they do, whether they develop mild or more severe COVID-19," according to Tropical Health Network.
Comments
Post a Comment